Multiple Choice
A sample of an ideal gas is heated and its Kelvin temperature doubles. What happens to the average speed of the molecules in the sample?
A) it is 1/ 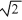 times smaller.
times smaller.
B) It doubles.
C) It halves.
D) it is 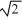 times as large.
times as large.
E) It does not change.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: How much compressional stress builds up
Q15: Avogadro's number has what units?<br>A) J/mol-K<br>B) It's
Q16: Two liters of a perfect gas are
Q17: A sample of a diatomic ideal gas
Q18: A sample of an ideal gas is
Q20: A mixture of helium and another gas
Q21: Consider an ideal gas at 1.0 atmosphere
Q22: At which of the following temperatures would
Q23: An ideal gas at STP is first
Q24: In order to double the average speed