Multiple Choice
If liquid ammonia and water were mixed, between which two atoms in these molecules would a hydrogen bond form?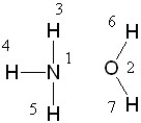
A) between 1 and 3
B) between 2 and 6
C) between 3 and 2, 4 and 2, 5 and 2, 1 and 6, and 1 and 7
D) Hydrogen bond formation is not possible between these two molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Which of the following terms does not
Q10: Which of the following compounds would have
Q11: Which of the following would have the
Q12: How many grams of Ag can be
Q13: The ClF molecule is polar. Use <sup>+</sup>
Q15: Which of the following statements concerning evaporation
Q16: In which of the following types of
Q17: Which of the following kinds of intermolecular
Q18: What is the final temperature after 336
Q19: Predict the predominate intermolecular force (London force,