Multiple Choice
Which of the following is the correct "set-up" for the problem "How many atoms are present in 15.0 g Na?"
A) 15.0 g Na × 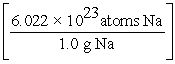
B) 15.0 g Na × × 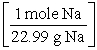
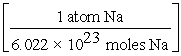
C) 15.0 g Na × × 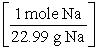
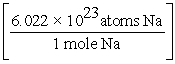
D) 15.0 g Na × 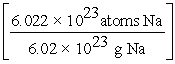
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: How many atoms of H are present
Q65: Contrast the two members of each pair
Q66: Contrast the two members of each pair
Q67: Analysis of a sample of a compound
Q68: Which of the following contains the greatest
Q70: Calculate the number of lithium atoms in
Q71: Which of the following quantities does not
Q72: The number of moles of O atoms
Q73: Calculate the percentage composition of oxygen in
Q74: The empirical formula of a compound is