Short Answer
The hypothetical element supposium exists in four isotopic forms. The relative masses and percentage abundances for these four isotopes are, respectively,
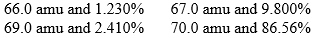
Calculate the atomic mass of supposium.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: The five naturally abundant isotopes of elemental
Q47: Fill in the blanks in the following
Q48: Match the molecules with the appropriate classification.<br>-
Q49: The total number of atoms present in
Q50: Which of the following statements is incorrect?<br>A)
Q52: What is the mass number of a
Q53: Isotopes of a given element _.<br>A) have
Q54: Which one of the following statements about
Q55: Match the molecules with the appropriate classification.<br>-
Q56: An atom contains 12 neutrons and 14