Short Answer
The hypothetical element athenium (Ah) occurs in two isotopic forms: 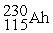 (86.500% abundance, relative mass of 230.145 amu) and
(86.500% abundance, relative mass of 230.145 amu) and 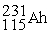 (13.500% abundance, relative mass of 231.769 amu). Calculate the atomic mass of athenium.
(13.500% abundance, relative mass of 231.769 amu). Calculate the atomic mass of athenium.
Correct Answer:

Verified
0.86500 x 230.145 amu = 199.07...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q33: Which of the following represents a pair
Q34: The number of electrons and neutrons in
Q35: Match the molecules with the appropriate classification.<br>-
Q36: In the formula C<sub>4</sub>H<sub>8</sub>O<sub>2</sub> _.<br>A) there are
Q37: Place in the blank the letter of
Q39: Which of the following pairs of atoms
Q40: The nucleus of an atom _.<br>A) contains
Q41: Fill in the blanks in the following
Q42: Depicted below is a discharge tube. The
Q43: Fill in the chart below for each