When Temperature Is Held Constant, the Pressure And Volume Of a Quantity of Gas Are Inversely Proportional (Boyle's Law)
Short Answer
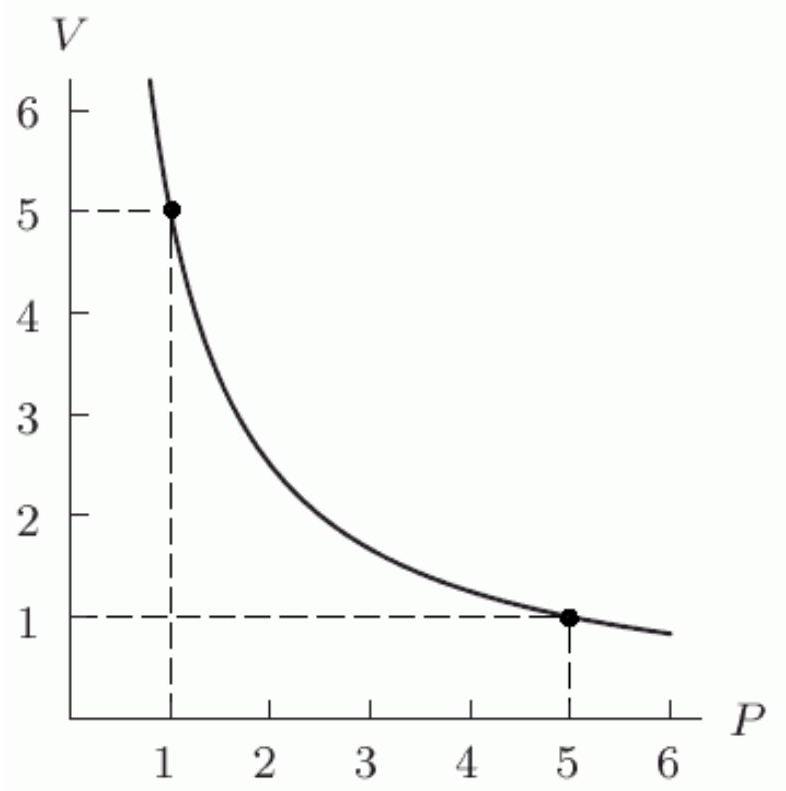
When temperature is held constant, the pressure and volume of a quantity of gas are inversely proportional (Boyle's Law). The following figure shows this relationship for a particular gas. Find a formula for in terms of and use it to find when is 7 . Round to 2 decimal places.
Correct Answer:

Verified
Correct Answer:
Verified
Q79: The figure below shows the graphs
Q80: The volume occupied by a fixed quantity
Q81: What is the long-run behavior of
Q82: A <span class="ql-formula" data-value="12 \mathrm{~kg}"><span
Q83: Can <span class="ql-formula" data-value="f(x)=x^{3}\left(x^{4}\right)^{4}"><span class="katex"><span
Q85: Which of the following statements are
Q86: The power function through the points
Q87: Which of the following statements are
Q88: The domain of the function
Q89: Use a graphing calculator or computer