Essay
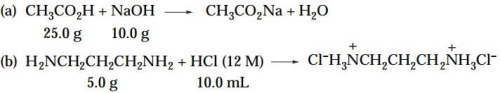
For each of the following reactions, (1) identify the limiting reagent, and (2) calculate the theo- retical yield of the organic product. (Note: The equations as shown are not necessarily bal- anced.)
Correct Answer:

Verified
(a) moles of acetic acid = (25.0 g) / (6...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: What would be the heat source (or
Q7: What should you do in each of
Q8: Find the following items in your student
Q9: Find the hazard information for each of
Q10: Give reasons for the following safety rules.<br>(a)
Q11: At the end of a reaction, your
Q13: You open your laboratory drawer to prepare
Q14: Make the following conversions.5.0 g CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>Br to
Q15: You need 45 mL of a 5%
Q16: Calculate the percent yield when:<br>(a) the theoretical