Multiple Choice
What is the slow, rate-determining step, in the acid-catalyzed dehydration of 2-methyl-2-propanol?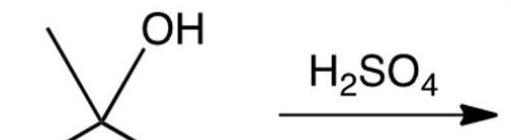
A) Protonation of the alcohol to form an oxonium ion.
B) Loss of water from the oxonium ion to form a carbocation.
C) Loss of a -hydrogen from the carbocation to form an alkene.
D) The simultaneous loss of a -hydrogen and water from the oxonium ion.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which of the following would have the
Q5: Which of the alkenes below has the
Q6: Which of the following alkenes exhibit
Q7: Which of the following stereoisomers gives the
Q8: Carbon-carbon double bonds do not freely
Q10: Identify the major organic product expected from
Q11: Which of the following compounds gives
Q12: If you wanted to make compound
Q13: Which of the following carbocations is(are) likely
Q14: What is the IUPAC name of the