Multiple Choice
Based on the van der Waals equation of state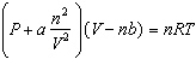
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.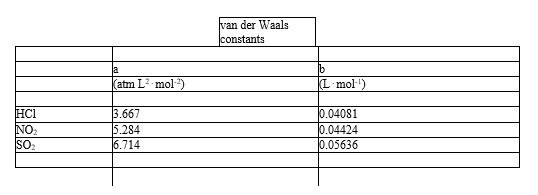
A) HCl
B) NO2
C) SO2
D) Must know n to answer this question
Correct Answer:

Verified
Correct Answer:
Verified
Q1: At 20°C water has a vapor pressure
Q2: Which of the following is a primary
Q3: With respect to the Maxwell-Boltzmann probability distribution
Q4: Name the following compound. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10703/.jpg" alt="Name
Q5: Arrange the follow in order of increasing
Q6: A gas has a temperature of 34.9°C
Q8: Which of the following molecules is chiral<br>A)<br><img
Q9: The vibration frequency of HCl is 8.66´10<sup>13</sup>
Q10: Helium effuses through a small opening at