Multiple Choice
The wavefunction for the 2s orbital in hydrogen is given by 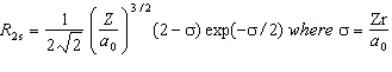 At what radius is there a node?
At what radius is there a node?
A) r = 0
B) r = 2/a0
C) r = a0
D) r = 2a0
E) there are no radial nodes in this function
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: A MO orbital for a heteronuclear diatomic
Q2: Given that Z<sub>eff</sub>(2s) in Li is 1.26,
Q4: Oxygen is paramagnetic and has a bond
Q5: Which of the following combinations of atomic
Q6: Of the following angular components for the
Q7: What do you predict of the electron
Q8: Rank from smallest to largest in terms
Q9: The molecule HeH<sup>+</sup> has an antibonding MO