Multiple Choice
Suppose the average thermal speed of molecules in a gas at room temperature is . If we "double" the gas's temperature (to ) , what is the average molecular speed now? Select the closest response.
A) About the same
B) 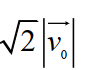
C) 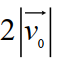
D) 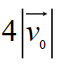
E) 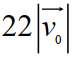
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q132: A person is sitting at rest on
Q133: Which of the following things is
Q134: Which of the lettered graphs below
Q135: Which of the following things is
Q136: Suppose we launch a disk so it
Q138: An object of mass <span
Q139: If the sound level increases by
Q140: Consider a collision where an object with
Q141: Two hockey pucks are initially at
Q142: Assume that <span class="ql-formula" data-value="D"><span