Multiple Choice
Suppose the interaction between two atoms has the effective potential energy curve shown below:
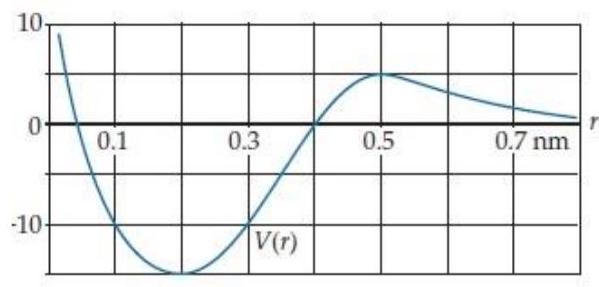
I have marked the vertical scale on this graph using an arbitrary energy unit we'll call bondits.
-Suppose instead we know that at the atoms are at rest at a separation of . What minimum energy is required to break the bond in this case?
A) Only a smidgen (less than a bondit)
B) 5 bondits
C) 10 bondits
D) 15 bondits
E) 20 bondits
F) The bond is already broken (or we can't form a bond) .
Correct Answer:

Verified
Correct Answer:
Verified
Q175: Which of the following frames would be
Q176: Suppose we put a <span
Q177: Suppose that <span class="ql-formula" data-value="\vec{u}"><span
Q178: An object of mass <span
Q179: A basketball bounces off the floor. It
Q181: An external interaction exerts a force
Q182: A baseball player slides into third base.
Q183: A cup sitting on a table constantly
Q184: The angular momentum (around a rotating rigid
Q185: Suppose an unpowered but moving railroad car