Multiple Choice
Imagine that the energy levels of a certain system are proportional to , where Imagine that for some reason, only transitions such that are physically possible. In a spectrum chart (like the one shown in figure Q11.2) the emission lines produced by this system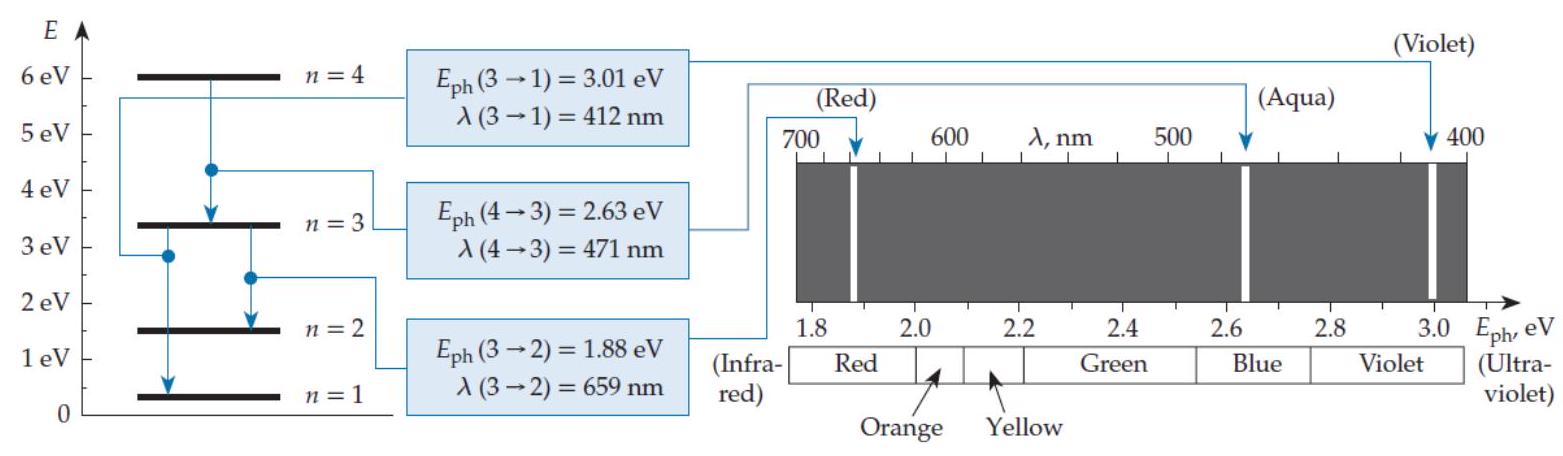
A) Get closer together (in energy) as we go to the right.
B) Are evenly spaced (in energy) .
C) Get farther apart (in energy) as we go to the right.
Correct Answer:

Verified
Correct Answer:
Verified
Q48: <span class="ql-formula" data-value="{ }_{26}^{55} \mathrm{Fe}"><span class="katex"><span class="katex-mathml"><math
Q49: Imagine we have an adjustable slit that
Q50: Suppose we create a traveling compression wave
Q51: A tilted top that is spinning clockwise
Q52: In a specific non-dispersive medium, a sinusoidal
Q54: The <span class="ql-formula" data-value="{ }_{2}^{6}"><span
Q55: A quanton is in a situation where
Q56: Consider an electron in a hydrogen
Q57: Suppose that at a certain instant
Q58: Suppose we have a quantum system