Multiple Choice
Why, primarily, does tetrahydrofuran (THF, bp 66 °C) have a higher boiling point than cyclopentane (bp 49 °C) ? 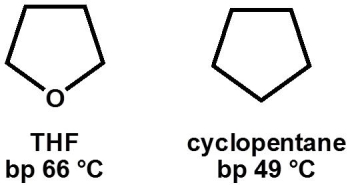
A) increased London dispersion forces
B) hydrogen bonding
C) increased mass
D) decreased London dispersion forces
E) dipole-dipole interactions
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following is a tertiary
Q2: Choose the best name for the following
Q3: How many of the nine constitutional isomers
Q5: Which of the following trimethylcyclohexanes has the
Q6: Choose the best name for the following
Q7: Which of the following names is correct?
Q8: Which of the following dimethylcyclohexanes has the
Q9: Rank the alcohols, all of similar molecular
Q10: Choose the best name for the following