Multiple Choice
Which of the following acid-base reactions favor the products?
A) HF + H2O ? F- + H3O+
B) 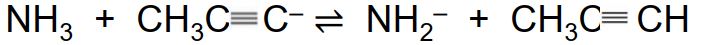
C) CH3CH3 + H2C=CH- ? CH3CH2- + H2C=CH2
D) CH3CO2H + CH3CH2O- ? CH3CO2- + CH3CH2OH
E) CH3OH + CH3CO2H ? CH3OH2+ + CH3CO2-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which compound is the conjugate base of
Q2: Which of the following is a Lewis
Q3: Which of the following is a factor
Q4: Which of the following is the strongest
Q5: Consider the following equilibrium:<br>CH<sub>3</sub>C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBP1106/.jpg" alt="Consider
Q6: Which of the following is the most
Q7: What form of glutamic acid (below) predominates
Q9: Which of the following is a Lewis
Q10: Which of the following is not a