Multiple Choice
A titration curve for a weak acid is shown.Which point shows the most buffering? 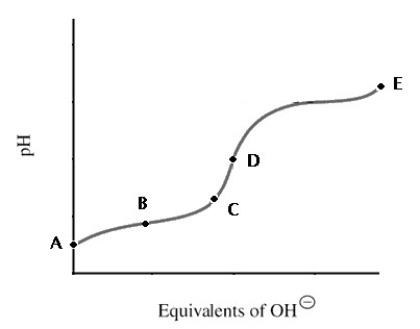
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: The ion-product constant for water,Kw,is<br>A)1 × 10-⁷
Q57: The self-ionization of water is _.<br>A)a unimolecular
Q58: Which statement explains the cleaning action of
Q59: The buffering capacity of a weak acid
Q60: The abundance of water in the cells
Q62: Since the pKa of acetic acid is
Q63: Proteins dissolved in water can be hydrolyzed
Q64: A solution containing 10-⁸ M HCl and
Q65: Which would you expect to be most
Q66: In the detergent sodium dodecyl sulfate,the sulfate