Multiple Choice
Refer to the following figure to answer the following questions.
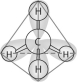
-In the methane molecule shown here, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electrons in these bonds are said to have
A) double orbitals.
B) tetrahedral orbitals.
C) complex orbitals.
D) hybrid orbitals.
E) reduced orbitals.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: What is the difference between covalent bonds
Q33: Calcium has an atomic number of 20
Q34: A covalent bond is likely to be
Q35: Please refer to Figure 2.1 to
Q36: Please refer to Figure 2.1 to
Q38: The nucleus of a nitrogen atom contains
Q38: What is the maximum number of covalent
Q39: The hybrid orbitals in a molecule of
Q40: Use the information extracted from the
Q41: The atomic number of neon is 10.