Multiple Choice
Refer to the following figure to answer the following questions.
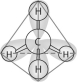
-Which of the following pairs of atoms would be most likely to form a covalent bond?
A) 
B) 
C) 
D) 
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: A covalent chemical bond is one in
Q22: Refer to the following figure to answer
Q23: Different atomic forms of an element contain
Q25: A van der Waals interaction is the
Q27: A molecule of carbon dioxide (CO₂)is formed
Q28: Which of the following statements correctly describes
Q29: From its atomic number of 15, it
Q30: Use the information extracted from the periodic
Q31: Sometimes atoms form molecules by sharing two
Q49: The atomic number of chlorine is 17.