Multiple Choice
Refer to the following figure to answer the following questions.
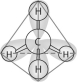
-Which of the following pairs of atoms would be most likely to form an ionic bond?
A) 
B) 
C) 
D) 
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the following molecules contains the
Q2: An atom with an atomic number of
Q3: What coefficients must be placed in the
Q6: Each element is unique and different from
Q7: The ionic bond of sodium chloride is
Q8: Please refer to Figure 2.1 to answer
Q9: <span class="ql-formula" data-value="3"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mn>3</mn></mrow><annotation encoding="application/x-tex">3</annotation></semantics></math></span><span
Q10: About 25 of the 92 natural elements
Q23: Which of the following explains most specifically
Q73: A group of molecular biologists is trying