Essay
Draw Lewis structures for each hypothetical molecule shown below, using the correct number of valence electrons for each atom. Determine which molecule makes sense because each atom has a complete valence shell and each bond has the correct number of electrons. Explain what makes the other molecules nonsensical, considering the number of bonds each type of atom can make.
a.
c.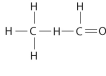
b.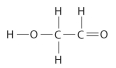
d. 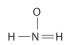
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The atomic number of sulfur is 16.
Q71: Oxygen has an atomic number of 8
Q72: Refer to the following figure to answer
Q73: Use the information extracted from the periodic
Q75: Use the information extracted from the periodic
Q77: Refer to the following figure to answer
Q78: How do isotopes of the same element
Q79: Use the information extracted from the periodic
Q80: Use the information extracted from the periodic
Q81: The mass number of an element can