Multiple Choice
The following questions are based on the reaction A + B → C + D shown in Figure 8.2.
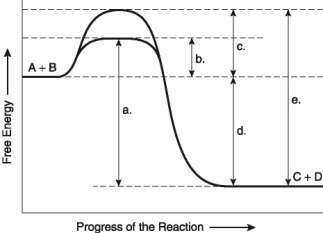
-Assume that the reaction has a △G of -5.6 kcal/mol. Which of the following would be True?
A) The reaction could be coupled to power an endergonic reaction with a △G of +6.2 kcal/mol.
B) The reaction could be coupled to power an exergonic reaction with a △G of +8.8 kcal/mol.
C) The reaction would result in a decrease in entropy (S) and an increase in the total energy content (H) of the system.
D) The reaction would result in an increase in entropy (S) and a decrease in the total energy content (H) of the system.
E) The reaction would result in products (C + D) with a greater free-energy content than in the initial reactants (A + B) .
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The following questions are based on the
Q3: The following questions are based on the
Q4: Which of the following statements regarding enzymes
Q5: What term is used to describe the
Q7: The following questions are based on the
Q8: Chemical equilibrium is relatively rare in living
Q9: The following questions are based on the
Q10: Which of the following is considered an
Q11: The following questions are based on the
Q34: Which of the following shows the correct