Multiple Choice
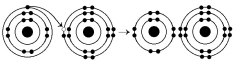
-What results from the chemical reaction illustrated above?
A) a cation with a net charge of +1
B) a cation with a net charge of -1
C) an anion with a net charge of +1
D) an anion with a net charge of -1
E) a cation with a net charge of +1 and an anion with a net charge of -1
Correct Answer:

Verified
Correct Answer:
Verified
Q6: About 25 of the 92 natural elements
Q7: One difference between carbon-12( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt="One
Q8: What bonding or interaction is most likely
Q9: A covalent bond is likely to be
Q10: Two atoms appear to have the same
Q12: If a salamander relied on hydrogen bonds
Q13: Which of the following best describes the
Q14: The atomic number of nitrogen is 7.
Q15: An atom has 6 electrons in its
Q16: In the term trace element, the modifier