Multiple Choice
Identical heat lamps are arranged to shine on identical containers of water and methanol (wood alcohol) , so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
A) 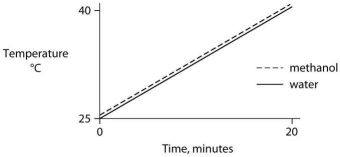
B) 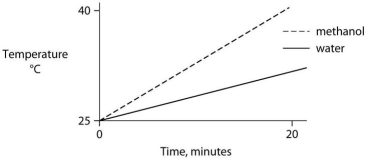
C) 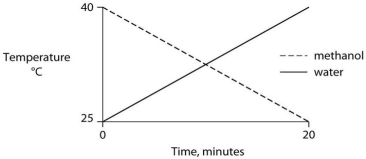
D) 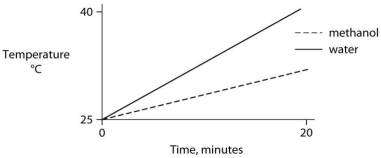
E) 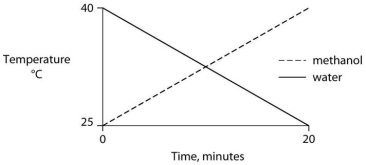
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Use the following information to answer the
Q20: The biological significance of water being its
Q21: We can be sure that a mole
Q23: If the cytoplasm of a cell is
Q32: Carbon dioxide (CO₂)is readily soluble in water,
Q41: What is the pH of a solution
Q43: Water molecules are able to form hydrogen
Q56: You have a freshly prepared 1 M
Q67: If the pH of a solution is
Q70: A beaker contains 100 mL of NaOH