Multiple Choice
The following questions are based on the reaction A + B ↔ C + D shown in the figure below.
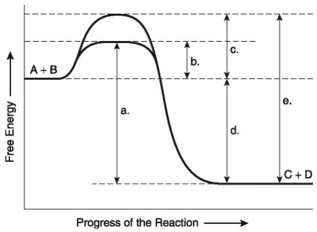
-Assume that the reaction in the figure above has a ΔG of -5.6 kcal/mol. Which of the following would be true?
A) The reaction could be coupled to power an endergonic reaction with a ΔG of +6.2 kcal/mol.
B) The reaction could be coupled to power an exergonic reaction with a ΔG of +8.8 kcal/mol.
C) The reaction would result in a decrease in entropy (S) and an increase in the total energy content (H) of the system.
D) The reaction would result in an increase in entropy (S) and a decrease in the total energy content (H) of the system.
E) The reaction would result in products (C + D) .
Correct Answer:

Verified
Correct Answer:
Verified
Q15: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" Activity of
Q16: Which of the following types of reactions
Q17: Which of the following is (are)true for
Q27: Which of the following is most similar
Q34: When 10 000 molecules of ATP are
Q35: Which of the following is the smallest
Q47: The following questions are from the end-of-chapter
Q51: Mutations that result in single amino acid
Q52: Why is ATP an important molecule in
Q70: The mathematical expression for the change in