Multiple Choice
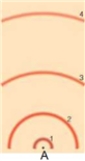
-In the above model of an atom, if you were to draw an electron's transition from the second excited state to the third excited state, what numbers would this correspond from/to What kind of spectrum would be produced
A) 3 to 4, absorption
B) 2 to 3, absorption
C) 3 to 4, emission
D) 2 to 3, emission
Correct Answer:

Verified
Correct Answer:
Verified
Q70: The lowest energy level in an atom
Q71: When an electron transitions from a higher
Q72: If continuous spectrum light from a star
Q73: The Doppler effect is sensitive only to
Q74: Two white dwarf stars are the same
Q76: To create the above spectra, which of
Q77: Electrons in an atom are attracted to
Q78: If temperature refers to the intensity of
Q79: The _ is responsible for binding the
Q80: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5757/.jpg" alt=" -In the above