Not Answered
in the following model of an atom, draw a transition from the ground state to the first excited state. To draw this, draw the electron's motion as a line with an arrow to indicate direction and draw the photon as a wave with an arrow to indicated direction. Label the electron and photon. Label whether this is an absorption, emission, or continuous spectrum. 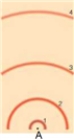
Correct Answer:

Verified
Correct Answer:
Verified
Q79: The _ is responsible for binding the
Q80: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5757/.jpg" alt=" -In the above
Q81: _ has a negative charge and a
Q82: A zero Kelvin temperature is also known
Q83: Since an electron is so less massive
Q85: What is the order of star colors
Q86: _ is the agitation among the molecules
Q87: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5757/.jpg" alt=" -Which series is
Q88: Figure 6-1<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5757/.jpg" alt="Figure 6-1
Q89: Suppose the laboratory wavelength of a spectral