Not Answered
in the following model of an atom, draw a transition from the second excited state to the first excited state. To draw this, draw the electron's motion as a line with an arrow to indicate direction and draw the photon as a wave with an arrow to indicated direction. Label the electron and photon. Label whether this is an absorption, emission, or continuous spectrum. 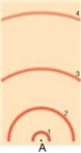
Correct Answer:

Verified
Correct Answer:
Verified
Q115: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5757/.jpg" alt=" -In the above
Q116: If one star has a temperature of
Q117: Which of the following can be determined
Q118: The process of making an electron less
Q119: The longer the wavelength, the bluer and
Q120: The temperature of an object from which
Q121: Describe two ways in which an atom
Q123: The radiation emitted from a star has
Q124: An atom that has lost an electron
Q125: Which one of the hydrogen atoms below