Not Answered
in the following model of an atom, draw a transition from the first excited state to the third excited state. Then draw a transition from the third excited state to the second excited state. Then draw a transition from the second excited state to the first excited sate. To draw these, draw the electron's motion as a line with an arrow to indicate direction and draw the photon as a wave with an arrow to indicated direction. Label the electron and photon. Label whether this is an absorption, emission, or continuous spectrum. 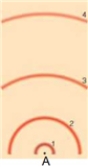
Correct Answer:

Verified
Correct Answer:
Verified
Q52: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5757/.jpg" alt=" -In the above
Q53: The Lyman series lines of hydrogen all
Q54: What would the spectrum of hydrogen look
Q55: When the electrons in an atom are
Q56: The hotter an object, the bluer it
Q58: If the radial velocity of a star
Q59: Red and blue in redshift and blueshift
Q60: Part of astrophysics is understanding the history
Q61: Why should photons emitted by a hotter
Q62: An atom that is excited<br>A)is also ionized.<br>B)is