Multiple Choice
Based only on the following illustration,it could be predicted that ice floats on liquid water because 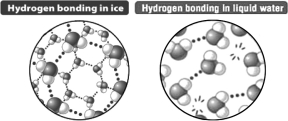
A) the crystal structure of ice is more regular than that seen in liquid water.
B) the distance between water molecules in ice is greater than in liquid water.
C) the cool temperature of ice reduces the extent of molecular motion relative to liquid water.
D) when ice forms, the hydrogen bond in the water molecule becomes nonpolar; ice behaves like oil.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The sum of an atom's protons and
Q5: ATP is a universal fuel for living
Q6: The monomers in proteins are _.
Q7: Which of the following is NOT a
Q8: An acid is a polar substance that
Q10: The most versatile atom in living systems
Q11: Of the following pH values,which indicates the
Q12: A molecule composed of amino acids is
Q13: Chemical reactions rearrange atoms,but do not create
Q14: The process of partial hydrogenation turns liquid