Multiple Choice
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
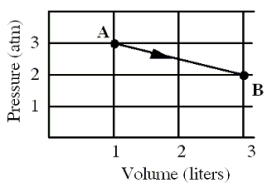
-What is the change in the internal energy,in calories,of the gas for this process?
A) zero calories
B) +12 cal
C) -110 cal
D) +110 cal
E) +122 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: Two moles of an ideal gas have
Q60: 5.00 kg of liquid water is heated
Q61: A Carnot engine has a heat input
Q62: A quantity of carbon monoxide gas is
Q63: Two moles of a confined ideal monatomic
Q65: A gasoline engine with an efficiency of
Q66: A heat engine operates between a hot
Q67: What change in temperature occurs when 1600
Q68: An ideal monatomic gas expands isobarically from
Q69: A container holding 1.2 kg of water