Multiple Choice
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
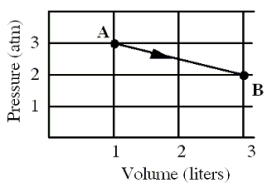
-How much work,in calories,was done by the gas?
A) zero calories
B) +12 cal
C) -110 cal
D) +110 cal
E) +121 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: A Carnot heat engine is to be
Q22: 5.00 kg of liquid water is heated
Q23: Determine the quantity of heat added to
Q24: A paddle wheel frictionally adds thermal energy
Q25: In an isothermal and reversible process,945 J
Q27: An ideal monatomic gas expands isothermally from
Q28: Heat is added to a sample of
Q29: An ideal monatomic gas expands isothermally from
Q30: A paddle wheel frictionally adds thermal energy
Q31: A container holding 1.2 kg of water