Multiple Choice
Assign the appropriate labels to the phase diagram shown below. 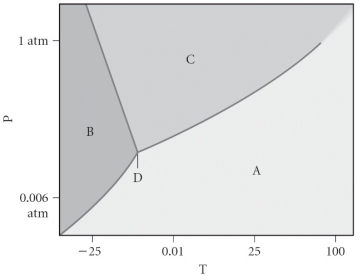
A) A = liquid, B = solid, C = gas, D = critical point
B) A = gas, B = solid, C = liquid, D = triple point
C) A = gas, B = liquid, C = solid, D = critical point
D) A = solid, B = gas, C = liquid, D = supercritical fluid
E) A = liquid, B = gas, C = solid, D = triple point
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Determine the vapour pressure (in mbar) of
Q25: Place the following substances in order of
Q40: Which of the following compounds has the
Q41: Give the change in condition to go
Q47: Match the following.<br><br>-CH<sub>2</sub>F<sub>2</sub><br>A)dispersion forces<br>B)dipole-dipole forces<br>C)ion-dipole forces<br>D)hydrogen bonding<br>E)ionic
Q49: Calculate the total quantity of heat required
Q75: Choose the substance with the lowest surface
Q91: Which of the following substances should have
Q92: Which of the following is considered a
Q119: Choose the substance with the highest vapour