Multiple Choice
Consider the phase diagram below. If the dashed line at 1 atm of pressure is followed from 100 to 500 °C, what phase changes will occur (in order of increasing temperature) ? 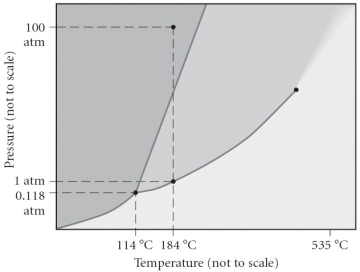
A) condensation followed by vaporization
B) sublimation followed by deposition
C) vaporization followed by deposition
D) fusion followed by vaporization
E) No phase change will occur under the conditions specified.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Why is the Δ<sub>vap</sub>H higher than Δ<sub>fus</sub>H<sub>
Q16: Give the term for the temperature at
Q36: Which of the following statements is FALSE?<br>A)
Q70: Which one of the following has a
Q89: What is the strongest type of intermolecular
Q104: Which is expected to have the largest
Q108: Choose the substance with the lowest vapour
Q113: Determine Δ<sub>vap</sub>H for a compound that has
Q123: Identify the substance with the highest viscosity
Q133: The freezing point of water is _.<br>A)