Multiple Choice
Determine the radius of an Al atom (in pm) if the density of aluminum is 2.71 g cm-3. Aluminum crystallizes in a face-centred cubic structure with an edge length of 2 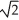 r.
r.
A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: NaCl crystallizes in a cubic unit cell
Q23: Lithium crystallizes in a body-centred cubic structure.What
Q36: Choose the substance with the highest viscosity
Q41: Give the change in condition to go
Q47: How much energy is required to vaporize
Q49: Calculate the total quantity of heat required
Q51: Based on the figure above, the boiling
Q55: Based on the figure above, the boiling
Q57: Identify the term used to describe the
Q64: How many H<sup>-</sup> ions are around each