Multiple Choice
Vanadium crystallizes in a body-centred cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium if the edge length of a bcc structure is 4r/ 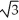 .
.
A) 3.06 g cm-3
B) 12.2 g cm-3
C) 6.11 g cm-3
D) 2.77 g cm-3
E) 8.46 g cm-3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: Define boiling.<br>A) A liquid becomes a gas.<br>B)
Q36: Choose the substance with the highest viscosity
Q42: Which of the following compounds exhibits only
Q50: Identify the compound that does not have
Q60: Based on the figure above, the boiling
Q64: How many H<sup>-</sup> ions are around each
Q99: Identify the compound that does not have
Q101: What is the edge length of a
Q102: Why do O, F and N, when
Q114: Place the following substances in order of