Multiple Choice
What is the hydronium ion concentration of a 0.500 mol L-1 acetic acid solution with Ka = 1.8 × 10-5? The equation for the dissociation of acetic acid is below: 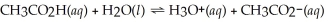
A) 3.0 × 10-2 mol L-1
B) 4.2 × 10-2 mol L-1
C) 3.0 × 10-3 mol L-1
D) 4.2 × 10-3 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Calculate the pH of a 1.60 mol
Q33: Calculate the hydroxide ion concentration in an
Q35: A 7.0 × 10<sup>-3</sup> mol L<sup>-1</sup> aqueous
Q37: Calculate the pH for an aqueous solution
Q39: Which one of the following will form
Q44: Determine the [OH⁻] concentration in a 0.169
Q84: Calculate the pOH of an aqueous solution
Q96: Determine the [OH<sup>-</sup>] concentration of a 0.116
Q120: Describe a molecule that can be a
Q121: Which of the following equilibria represents the