Multiple Choice
What is the hydronium ion concentration of a 0.150 mol L-1 hypochlorous acid solution with Ka = 3.5 × 10-8? The equation for the dissociation of hypochlorous acid is below: 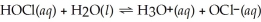
A) 1.9 × 10-4 mol L-1
B) 7.2 × 10-4 mol L-1
C) 2.8 × 10-5 mol L-1
D) 7.2 × 10-5 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q61: Do both protons ionize instantaneously from a
Q87: Determine the concentration of CO<sub>3</sub><sup>2-</sup> ions in
Q93: Determine the [OH<sup>-</sup>] concentration of a 0.741
Q108: Calculate the concentration of H<sub>3</sub>O⁺ in a
Q110: Which of the following solutions would have
Q111: Which Bronsted-Lowry acid is not considered to
Q116: Identify the weakest acid.<br>A)HF<br>B)HCl<br>C)HBr<br>D)HI<br>E)NaH
Q120: Determine the [OH⁻] concentration in a 0.235
Q121: List the compound that is formed from
Q154: Calculate the pH of a 0. 080