Multiple Choice
What is the pH of a 0.100 mol L-1 NH3 solution that has Kb = 1.8 × 10-5? The equation for the dissociation of NH3 is below: 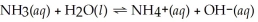
A) 1.87
B) 2.87
C) 11.13
D) 10.13
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Determine the pH in a 0.235 mol
Q22: Which of the following is a general
Q26: Which one of the following will form
Q31: Determine the pOH in a 0.235 mol
Q45: What is the pH of a 0.0
Q71: Calculate the pH of a 0.60 mol
Q72: Calculate the pH of a solution that
Q73: What is the pH of a 0.40
Q106: When dissolved in water,which compound is generally
Q126: Which of the following is a strong