Multiple Choice
What is the pH of a 0.30 mol L-1 pyridine solution that has Kb = 1.9 × 10-9? The equation for the dissociation of pyridine is below: 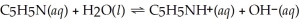
A) 4.62
B) 8.72
C) 9.38
D) 10.38
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Find the percent ionization of a 0.337
Q41: Calculate the molarity of hydroxide ion in
Q95: Identify the diprotic acid.<br>A) HNO<sub>3</sub><br>B) HCl<br>C) CH<sub>3</sub>COOH<br>D)
Q102: Calculate the hydroxide ion concentration in an
Q108: Calculate the concentration of H<sub>3</sub>O⁺ in a
Q109: Determine the pH of a 0.18 mol
Q113: Which of the following is a weak
Q135: Which of the following is the correct
Q152: Calculate the pH of a solution that
Q154: Calculate the pH of a 0. 080