Multiple Choice
Exhibit 2B Contains information on the pK's of some common buffers.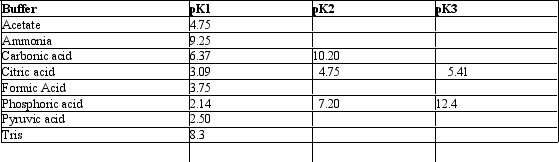 Refer to Exhibit 2B.An ammonium buffer would work well at this pH:
Refer to Exhibit 2B.An ammonium buffer would work well at this pH:
A) 5.6
B) 7.0
C) 9.0
D) 11.0
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: If the pH of 1 liter of
Q20: Molecules which contain both hydrophilic and hydrophobic
Q23: Exhibit 2A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1882/.jpg" alt="Exhibit 2A
Q32: What is the maximum number of hydrogen
Q37: Which of the following classes of compounds
Q61: When does a weak acid buffer best?<br>A)
Q79: What is the pH of an acetic
Q82: The ion product constant for water (K<sub>w</sub>)
Q83: In a hydrogen bond<br>A) three atoms lie
Q90: Which of the following molecules is polar?<br>A)