Multiple Choice
Exhibit 2B Contains information on the pK's of some common buffers.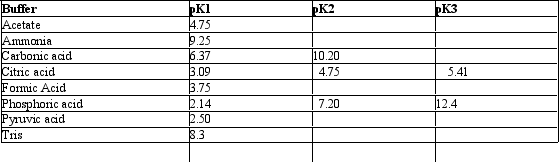 Refer to Exhibit 2B.A carbonate buffer would work well at this pH:
Refer to Exhibit 2B.A carbonate buffer would work well at this pH:
A) 4.0
B) 6.0
C) 8.0
D) 10.0
E) 6.0 and 10.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Which has the greater pK<sub>a</sub>, a weak
Q38: Which of the following classes of compounds
Q41: Which of the following molecules will not
Q42: An ammonia buffer contains NH<sub>3</sub>:NH<sub>4</sub><sup>+</sup> in a
Q56: Using the Henderson-Hasselbalch equation, calculate the pH
Q57: In a titration of a weak acid
Q58: Calculate the final pH of a solution
Q60: Exhibit 2A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1882/.jpg" alt="Exhibit 2A
Q68: For an acid that undergoes this
Q76: An HCl solution has a pH =