Multiple Choice
A 0.25 M aqueous solution of potassium chloride,KCl,is tested for conductivity using the type of apparatus shown.What do you predict will happen? 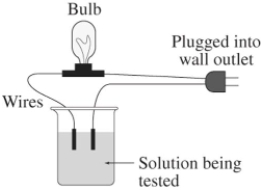
A) The bulb will not light up - KCl is a nonelectrolyte
B) The bulb will not light up - KCl is in the molecular form when dissolved in water
C) The light bulb will shine dimly - KCl is only partially ionized in aqueous solution
D) The light bulb will shine brightly - KCl is highly ionized in aqueous solution
Correct Answer:

Verified
Correct Answer:
Verified
Q30: What atmospheric component is responsible for the
Q31: Evaluate the ratio M<sub>H+</sub> (pH 3) /
Q32: Electronegativity<br>A)is a measure of an atom's attraction
Q33: A proton released by an acid in
Q34: The fact that carbon (C) is less
Q36: Predict the products of the chemical
Q37: A polar covalent bond is created when<br>A)a
Q38: Lakes surrounded by _ have very little
Q39: Which reaction represents an acid-base neutralization
Q40: A disadvantage of ozonation over chlorination is<br>A)the