Multiple Choice
A 0.25 M solution of the sugar sucrose,C12H22O11,in water is tested for conductivity using the type of apparatus shown.What do you predict will happen? 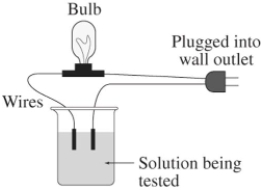
A) The bulb will not light up - sucrose is an electrolyte,but not very soluble in aqueous solution
B) The bulb will not light up - sucrose is in the molecular form in aqueous solution
C) The light bulb will shine dimly - sucrose is only partially ionized in aqueous solution
D) The light bulb will shine brightly - sucrose is highly ionized in aqueous solution
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Most aquatic life in lakes cannot survive
Q7: Which type of water may be considered
Q8: Which of the following is not a
Q9: Predict the products of this reaction:
Q10: Which concentration is consistent with a basic
Q12: What are the major disadvantages of using
Q13: Every increase of one pH unit indicates<br>A)an
Q14: Which is not a consequence of hydrogen
Q15: What is biological oxygen demand (BOD)?<br>A)A measure
Q16: Which of the following is not carried