Multiple Choice
Determine the energy change associated with the transition from 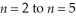 in the hydrogen atom.
in the hydrogen atom.
A) -2.18 × 10-19 J
B) +6.54 × 10-19 J
C) +4.58 × 10-19 J
D) -1.53 × 10-19 J
E) +3.76 × 10-19 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Calculate the wavelength (in nm)of a the
Q18: Choose the transition (in a hydrogen atom)below
Q19: _ are used to image bones and
Q20: Determine the mass of a ball with
Q21: How many different values of m<sub>l</sub> are
Q23: Place the following types of electromagnetic radiation
Q24: Identify the correct values for a 4f
Q27: Electromagnetic radiation with a wavelength of 575
Q33: Describe how a neon light works.
Q148: How many different values of m<sub>l</sub> are