Multiple Choice
Determine the energy change associated with the transition from 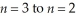 in the hydrogen atom.
in the hydrogen atom.
A) +3.03 × 10-19 J
B) -1.82 × 10-19 J
C) +5.51 × 10-19 J
D) -3.03 × 10-19 J
E) +2.69 × 10-19 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: On the electromagnetic spectrum,visible light is immediately
Q50: How many different values of m<sub>l</sub> are
Q51: Determine the shortest frequency of light required
Q52: Which of the following occur as the
Q53: What are the possible values of n
Q55: In which orbital would an electron (on
Q58: How much energy (in kJ)do 3.0 moles
Q59: Calculate the energy of the green light
Q69: What are the possible orbitals for n
Q112: Food can be cooked by _ radiation.<br>A)