Multiple Choice
Calculate the energy change associated with the transition from 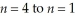 in the hydrogen atom.
in the hydrogen atom.
A) +4.89 × 10-18 J
B) +1.64 × 10-18 J
C) -6.12 × 10-18 J
D) +3.55 × 10-18 J
E) -2.04 × 10-18 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Which of the following statements is TRUE?<br>A)
Q51: Determine the shortest frequency of light required
Q55: In which orbital would an electron (on
Q58: How much energy (in kJ)do 3.0 moles
Q59: Calculate the energy of the green light
Q61: Describe the shape of a p orbital.<br>A)
Q63: Electromagnetic radiation with a wavelength of 640
Q63: Identify the color of a flame test
Q64: How many 8p orbitals exist?<br>A) 0<br>B) 1<br>C)
Q112: Food can be cooked by _ radiation.<br>A)