Multiple Choice
It is possible to determine the ionization energy for hydrogen using the Bohr equation.Calculate the ionization energy (in kJ) for a mole of hydrogen atoms,making the assumption that ionization is the transition from 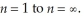
A) 7.62 × 103 kJ
B) 2.76 × 103 kJ
C) 1.31 × 103 kJ
D) 3.62 × 103 kJ
E) 5.33 × 103 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Which of the following statements is TRUE?<br>A)
Q23: Place the following types of electromagnetic radiation
Q24: Identify the correct values for a 4f
Q27: Electromagnetic radiation with a wavelength of 575
Q29: Give the value of l for a
Q31: Each of the following sets of quantum
Q33: Describe how a neon light works.
Q33: Consider a 3p orbital.How is it different
Q104: The vertical height of a wave is
Q148: How many different values of m<sub>l</sub> are