Multiple Choice
Calculate the energy change associated with the transition from 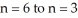 in the hydrogen atom.
in the hydrogen atom.
A) +5.35 × 10-19 J
B) -1.83 × 10-19 J
C) -2.42 × 10-19 J
D) -4.31 × 10-19 J
E) +6.86 × 10-19 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Determine the end (final)value of n in
Q49: Which of the following visible colors of
Q92: How many orbitals are contained in the
Q95: Which of the following statements is TRUE?<br>A)
Q116: Which of the following quantum numbers describes
Q131: Which of the following transitions (in a
Q133: In which orbital below would an electron
Q135: How many subshells are there in the
Q138: The de Broglie wavelength of an electron
Q141: Each of the following sets of quantum