Multiple Choice
Calculate the wavelength of light emitted from a mol of photons as they transition from 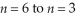 in the hydrogen atom.
in the hydrogen atom.
A) 1093 nm
B) 970 nm
C) 342 nm
D) 643 nm
E) 740 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Which of the following statements is TRUE?<br>A)
Q20: Determine the longest wavelength of light required
Q61: Describe the shape of a p orbital.<br>A)
Q63: Identify the color of a flame test
Q63: Electromagnetic radiation with a wavelength of 640
Q64: How many 8p orbitals exist?<br>A) 0<br>B) 1<br>C)
Q68: A green laser pointer has a wavelength
Q69: How many orbitals are associated with an
Q71: Which of the following transitions (in a
Q97: How many sublevels are contained in the