Multiple Choice
Pure acetic acid ( 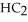
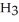
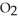 ) is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
) is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 15.00 mL of glacial acetic acid at 25°C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25°C is 1.05 g/mL.
A) 1.89 × 103 M
B) 31.5 M
C) 0.0315 M
D) 0.525 M
E) 5.25 × 10-4 M
Correct Answer:

Verified
Correct Answer:
Verified
Q40: How would the concentration change if a
Q71: Describe the difference between complete ionic and
Q123: How many moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6105/.jpg" alt="How
Q125: Match the following.
Q126: All of the following compounds are soluble
Q127: 94.20 mL of 0.800 M potassium hydroxide
Q131: How many liters of a 0.0550 M
Q133: Which of the following is a precipitation
Q167: Determine the molarity of a solution formed
Q218: Determine the oxidation state of Mn in